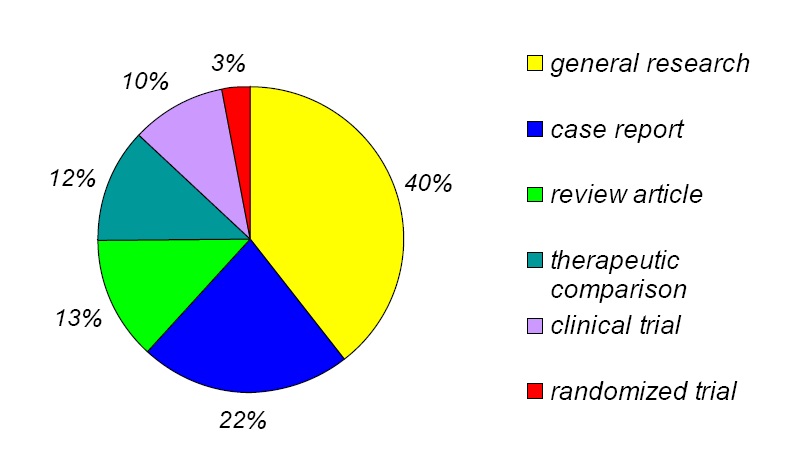
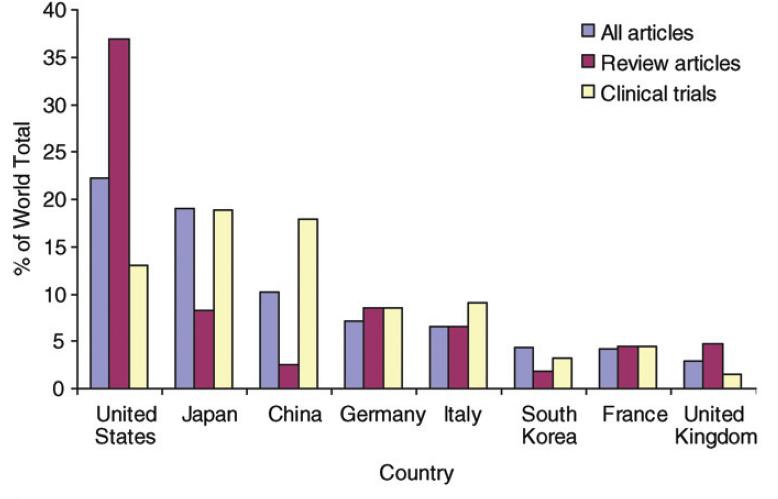
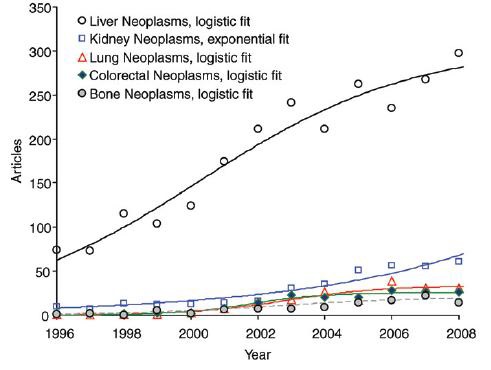
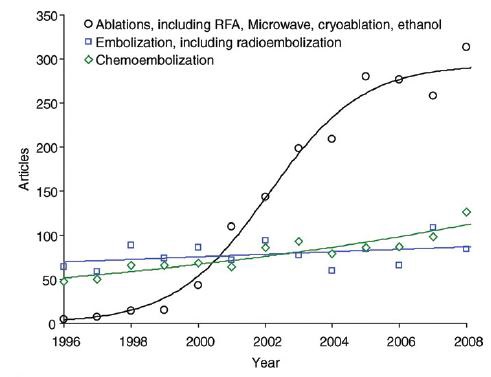
一、结直肠癌肝转移治疗概况
肿瘤介入放射学在美国,缓慢成长,被业界有限关注和仅获得低水平的基金
一、结肠癌肝转移介入治疗的历史 结直肠癌辅助化疗的历史始于1950~ 结肠癌术后化疗始于50年代应用塞替派(Thiotepa)开始。当时是由美国退伍军人管理局肿瘤外科组(Veterans Adminidtration Surgical Oncology Group ,VASOG)提出的。比较术后应用于不应用塞替派患者的5年生存率。1961和1962年发表两项多中心研究,1750例表明,5年生存率分别为55%和53%(1961)。未能证明塞替派对结直肠癌辅助治疗有效【35】。 早期临床试验未能证实包括塞替派、5-FU、FUDR和Me-CCNU等化疗药物与单纯手术切除对患者生存率的影响,两者没有显著的统计学差异。
1980~ 直到1988年,Buyse 等发表首个6800例结直肠癌辅助治疗的荟萃分析,证实虽与手术比较无统计学意义,但以5-FU为基础的辅助化疗可提高生存率2.3%-5.7%,而5年生存率就此提高到了5%的水平,中位生存期延长10个月。
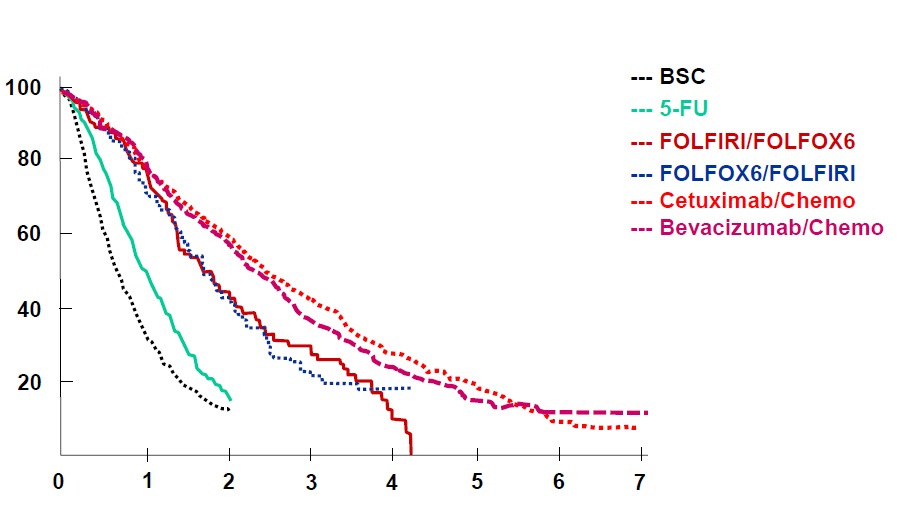
结直肠癌的生存率
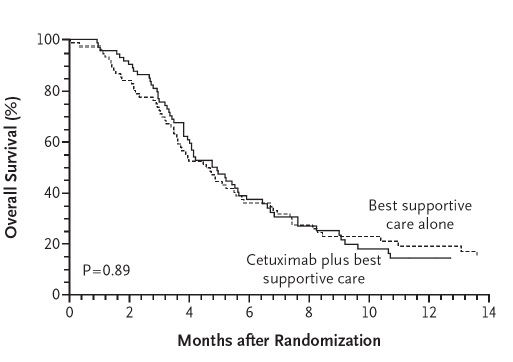
Colon Cancer Collaborative Group, BMJ 2000
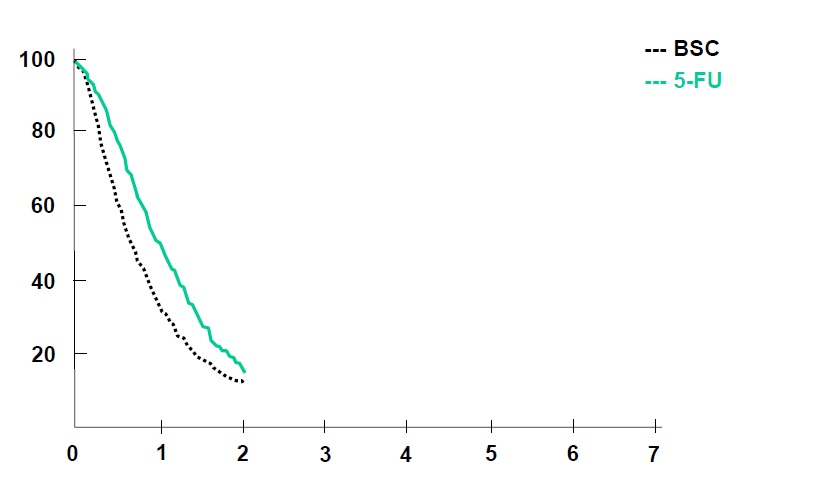
BSC=Best Surpport Care
Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000 Sep 2;321(7260):531-5.
同样是1988年,NSABP C-01的结果表明,Dukes′B期和C期结肠癌术后进行辅助化疗(MOF方案,379 例)与单纯手术(394 例)相比较,可改善 5 年生存率(67%vs.60%,P=0.07),此研究结果被后来发表的多中心研究证实。如5-FU/左旋咪唑(Levamisole,Lev)和 5-FU/醛氢叶酸(Leucovorin , LV)作为结肠癌术后辅助化疗方案,确实能提高无病生存率和总生存率。
1990~ NIH 于1990将5-FU/LV辅助化疗方案作为III期结肠癌术后的标准治疗。1994年,IMPACT的研究确定结肠癌术后应用5-FU/LV优于单独手术【4】。
2000~Mosaic III期临床试验,肯定了FOLOFX4>5-FU/LV辅助化疗方案
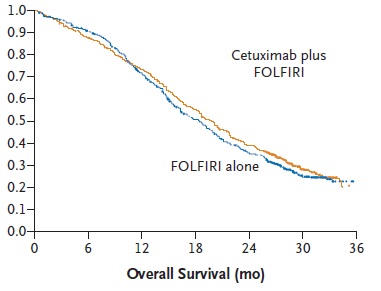
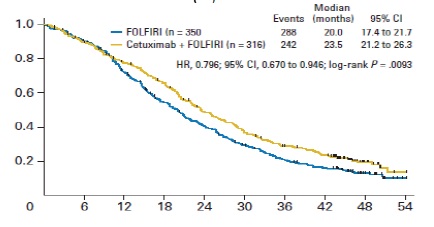
任何介入治疗手段都需要与目前结直肠肝转移的治疗的策略相比较,所以熟悉目前的非介入治疗的现状对于指导和了解介入治疗未来发展方向是十分重要的。 基本上现代结直肠癌肝转移的策略主要是从一线治疗到维持的策略 Colorectal cancer: survival 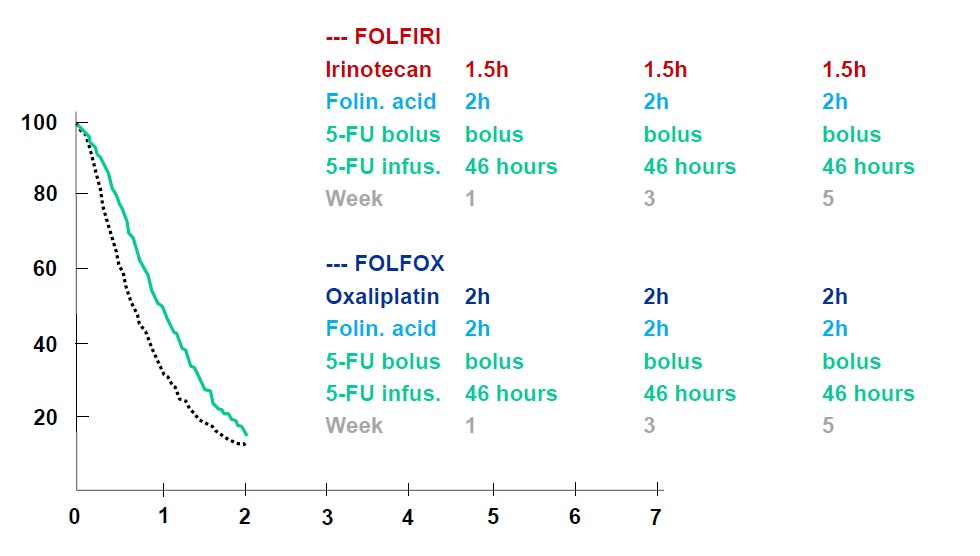 Colorectal cancer: survival 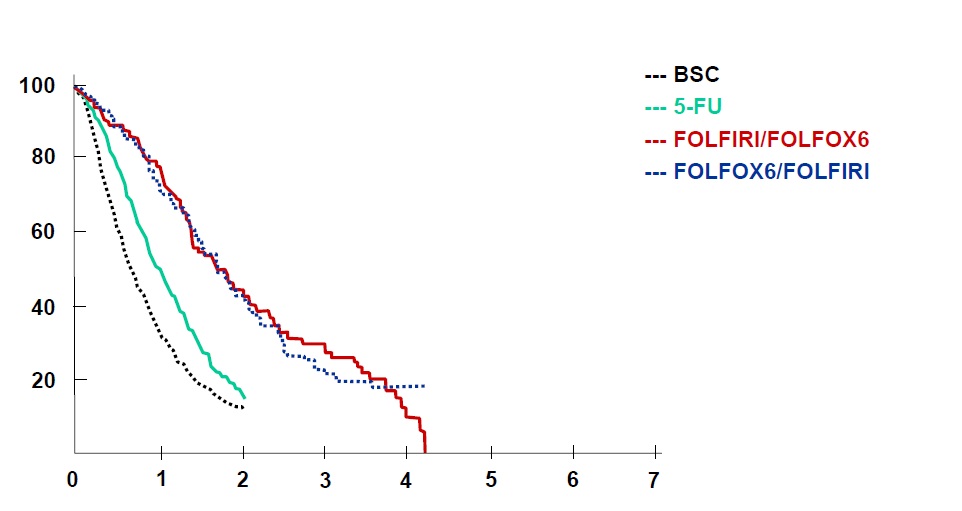
Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004 Jan 15;22(2):229-37. Epub 2003 Dec 2.
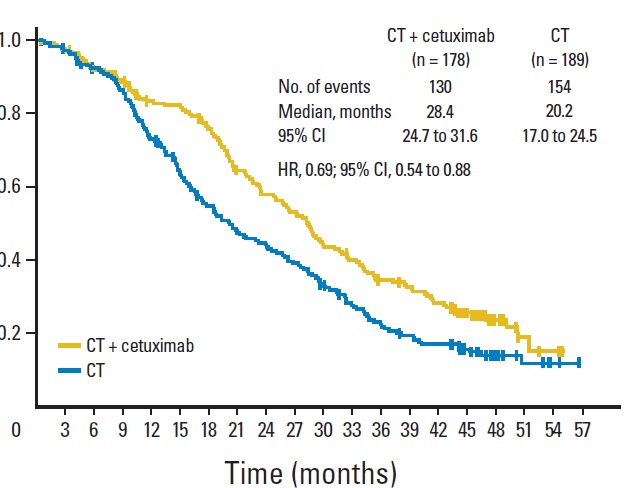
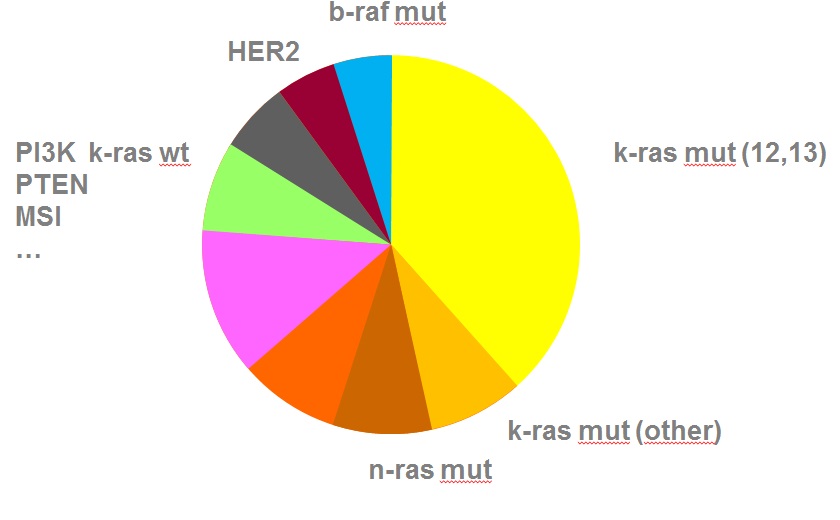
近年来,随着对细胞信号转导通路的认识,与CRC 相关的信号转导异常的研究也越来越深入,目前在对CRC 发生、发展的认识及其防治等方面取得了一定进展。在目前对CRC 的分子生物学及靶向治疗研究中,EGFR 和KRAS 基因是研究最为深入的靶点。
EGFR- antibodies and KRAS status 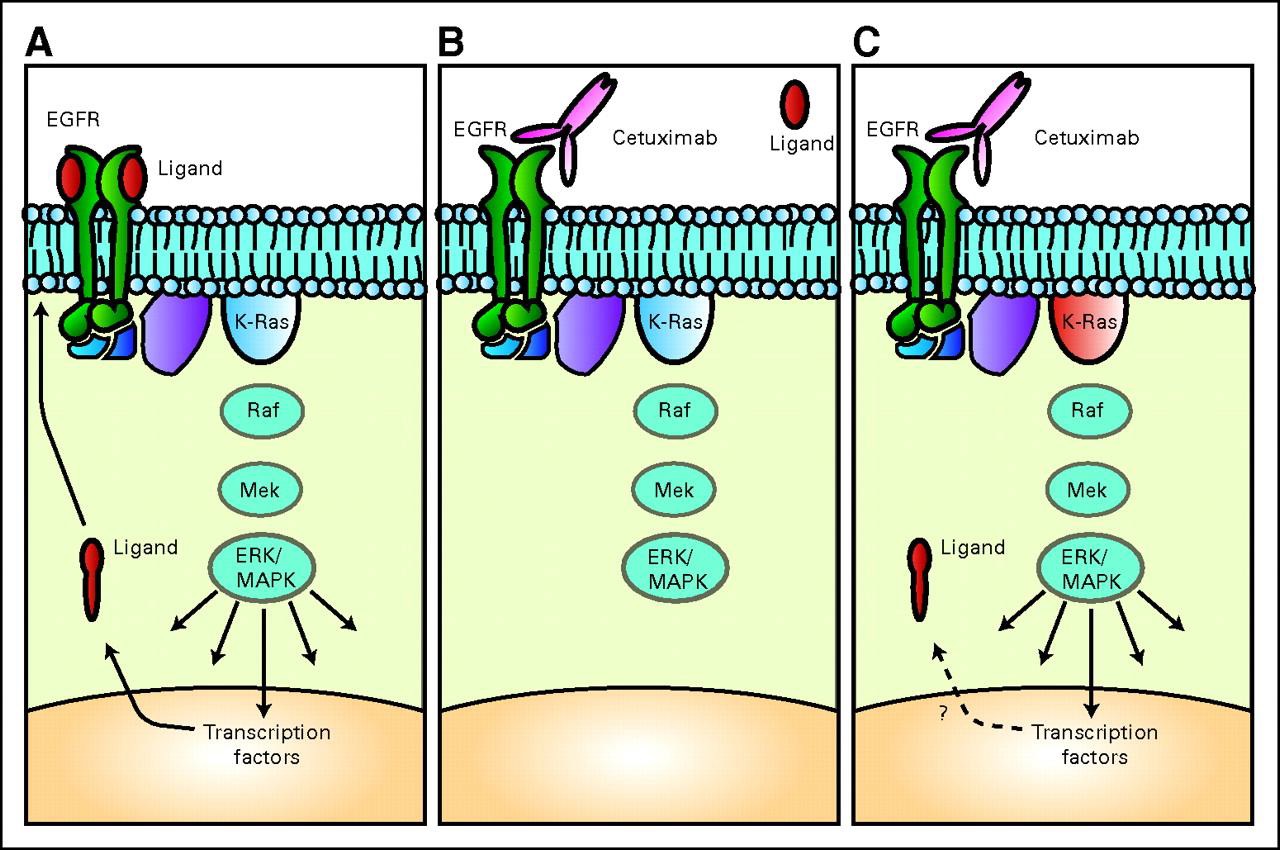 EGFR =epidermal growth factor receptor] 表皮生长因子受体 Ligand = 配体,同锚定蛋白结合的任何分子都称为配体(ligand) Cetuximab = 西妥昔单抗,西妥昔单抗 属于嵌合型IgG1单克隆抗体,分子靶点为表皮生长因子受体(EGFR)。EGFR信号途径参与控制细胞的存活,增殖、血管生成、细胞运动、细胞的入侵及转移等(百度)。
Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA 3rd, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007 Aug 1;25(22):3230-7.
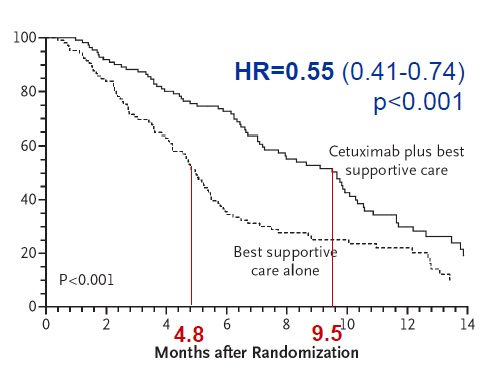
Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008 Oct 23;359(17):1757-65. doi: 10.1056/NEJMoa0804385.
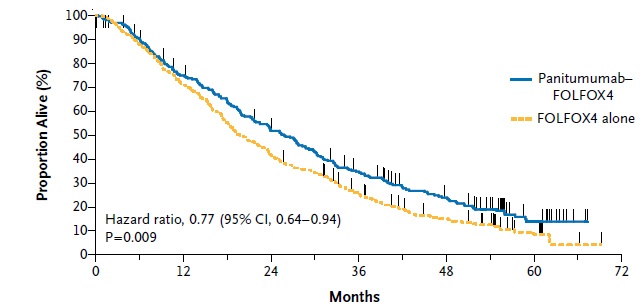
抗上皮生长因子作为一线治疗的临床实验
Anti - EGFR (1st line trials) , EGFR(Epidermal Growth Factor Receptor)上皮生长因子
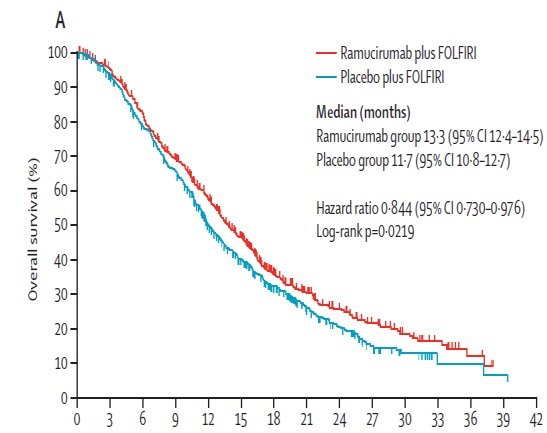
抗血管内皮生长因子二线治疗的临床实验
Ant i- VEGF (Receptor) (2nd line trials) VEGF,(vascular endothelial growth factor ,VEGF) 血管内皮生长因子
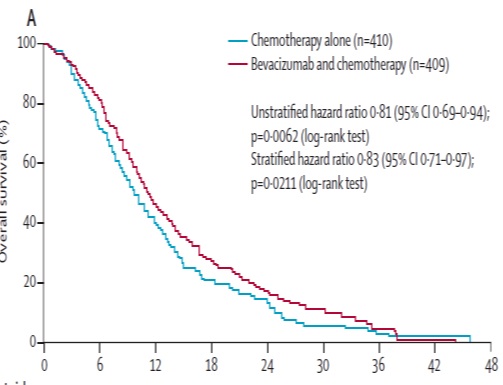
Anti-VEGF (1st line trials) 抗血管内皮生长因子一线治疗的临床实验
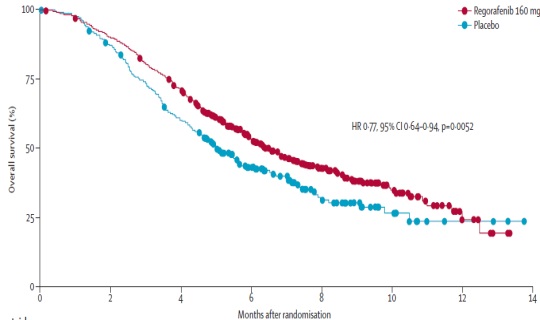
Anti-VEGF(R) (2nd line trials) 抗血管内皮生长因子作为二线治疗临床实验
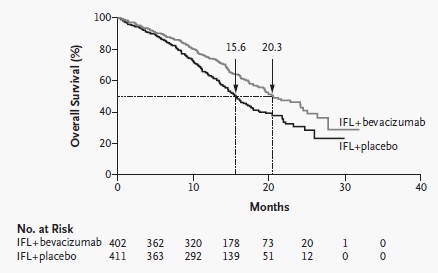
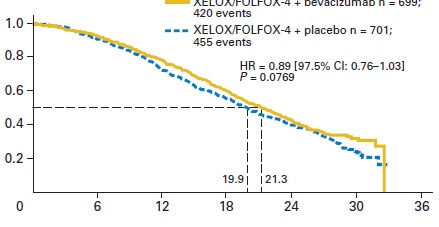
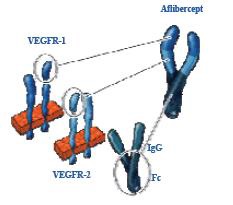
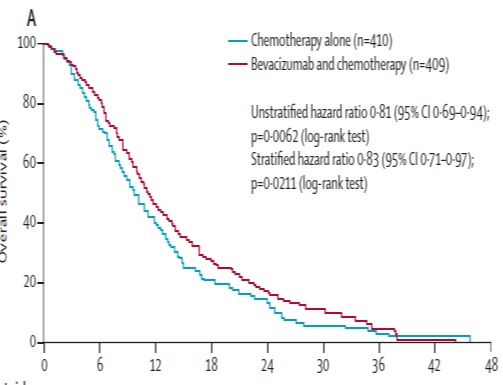
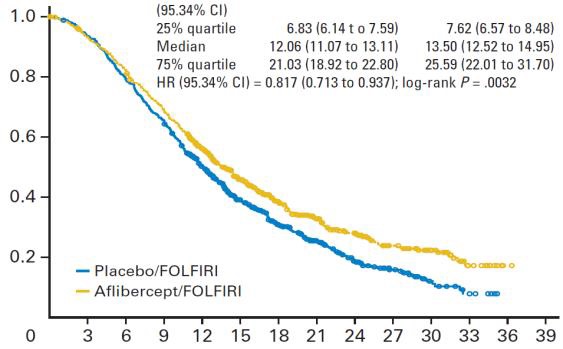
贝伐珠单抗(Bevacizumab,商品名Avastin)是一种单克隆抗体,可抑制血管内皮生长因子,用于治疗各类转移性癌症。 Aflibercept (阿柏西普):Zaltrap(Aflibercept)在以前治疗的转移性结肠直肠癌患者中显着改善生存,VELOR研究是一项跨国,随机,双盲试验,将FOLFIRI与Zaltrap或安慰剂联合用于治疗mCRC患者。该研究随机分配了1,226例以前用奥沙利铂治疗的mCRC患者。约30%的患者接受贝伐珠单抗治疗。主要终点是整体生存率的改善。次要终点包括无进展生存期,治疗反应和安全性。 Ramucirumab (雷莫芦单抗),人血管内皮生长因子受体2拮抗剂
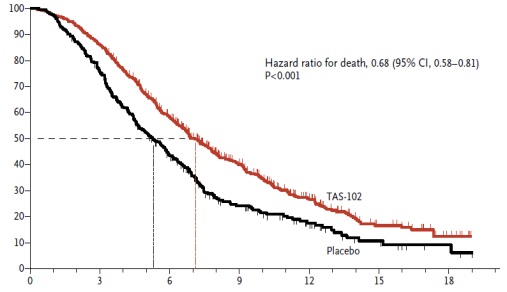
“Last” line
Colorectal cancer: survival
Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000 Sep 2;321(7260):531-5.(Colon Cancer Collaborative Group, BMJ 2000 )免费文献
Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A.FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004 Jan 15;22(2):229-37. Epub 2003 Dec 2. (Tournigand, JCO 2004 )免费文献
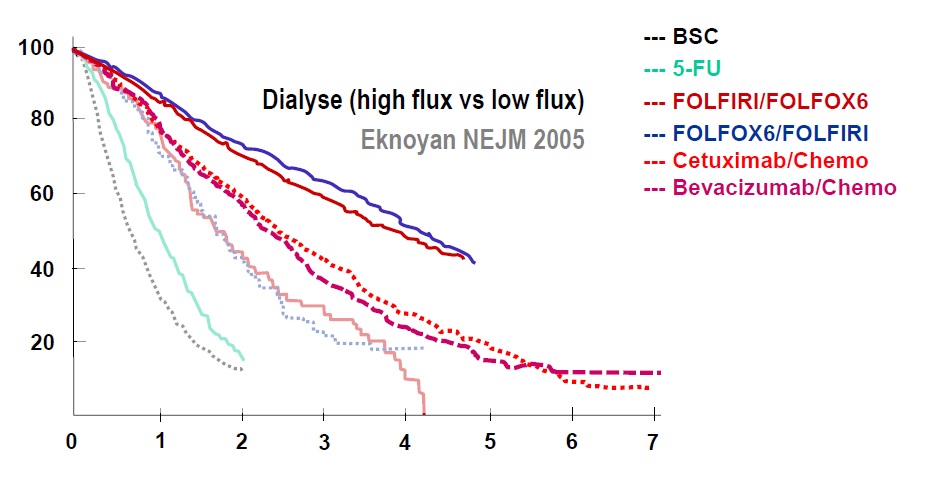
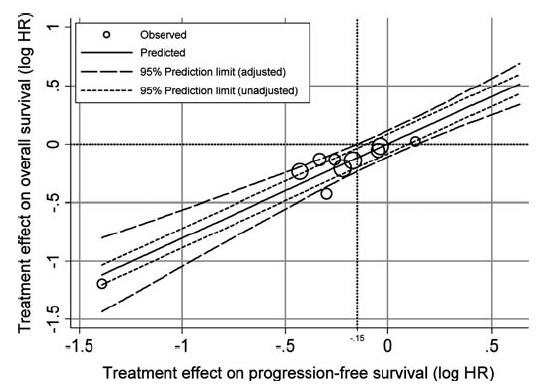
Venook ASCO 2014Eknoyan NEJM 2005(文献没找到)相关文献可能发表在NEJM 2002 PFS作为总生存的替代物(PFS as surrogate for Overall survival)
它本身是一个和时间无关的量。但是,相比于对中位生存时间进行T-检验或者线性回归,运用HR生存分析考虑了删失数据,且相对于单纯使用RR、OR或者是 Logistic回归而言,运用HR生存分析考虑了时间因素。进而能尽可能真实地反映该类数据所代表的结果。 Progression free survival (any site)
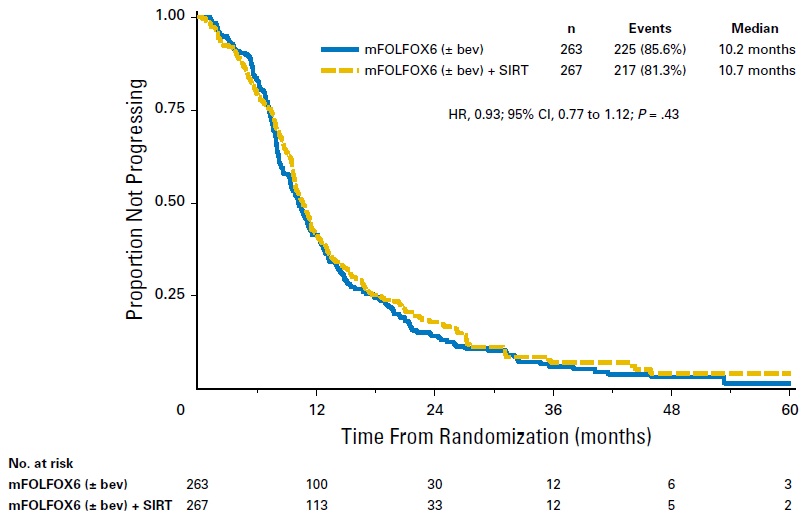
van Hazel GA at al,SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016 May 20;34(15):1723-31. doi: 10.1200/JCO.2015.66.1181. Epub 2016 Feb 22.
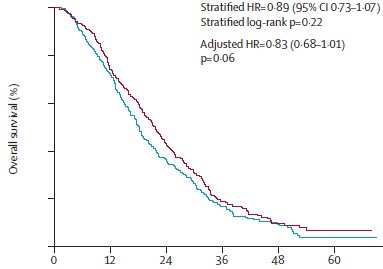
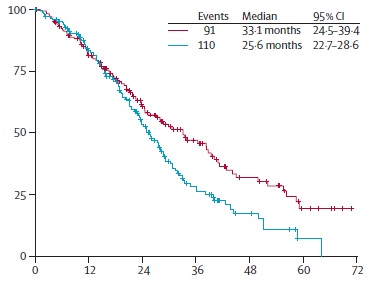
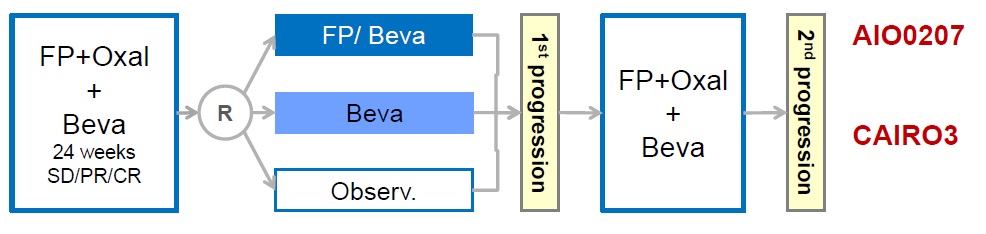 AIO OVERALL SURVIVAL
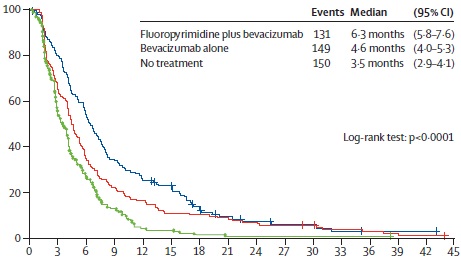
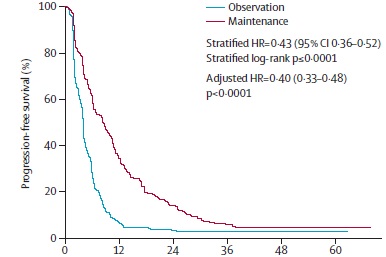
Hegewisch-Becker S at al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015 Oct;16(13):1355-69. doi: 10.1016/S1470-2045(15)00042-X. Epub 2015 Sep 8.
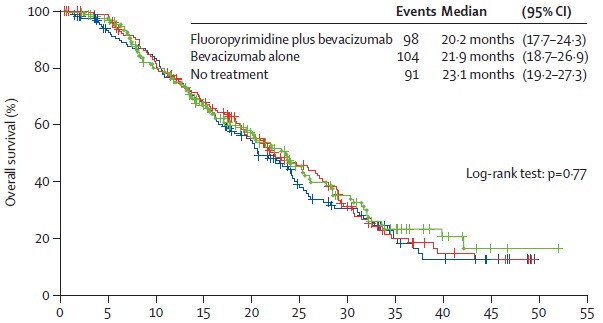
CAIRO3 OVERALL SURVIVAL
Simkens LH at al Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015 May 9;385(9980):1843-52. doi: 10.1016/S0140-6736(14)62004-3. Epub 2015 Apr 7.
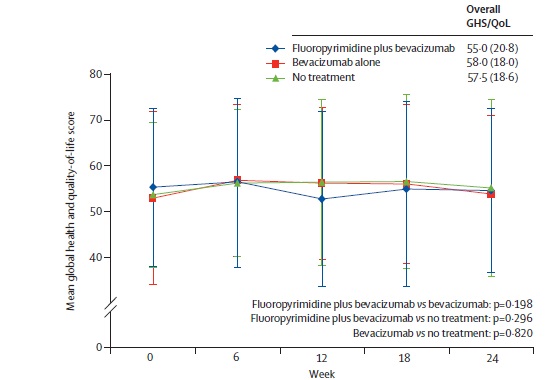
Quality of Life
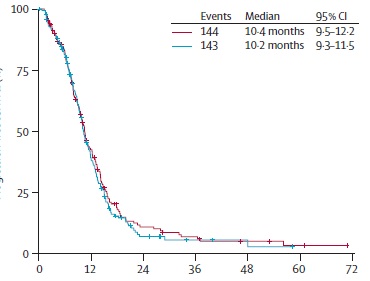
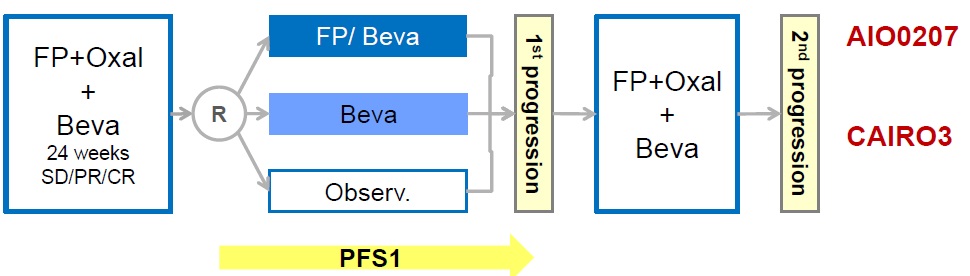 AIO progress free survival
CELIM: Blinded surgical review
Folprecht G at al Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010 Jan;11(1):38-47. doi: 10.1016/S1470-2045(09)70330-4. Epub 2009 Nov 26.
Response and resection rates within the trials 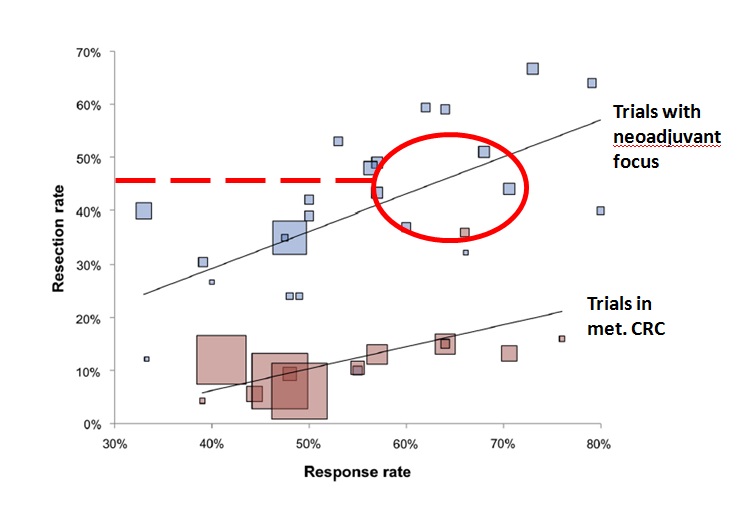
Folprecht G at al Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. 2014 May;25(5):1018-25.
Jones RP, Hamann S, Malik HZ3, Fenwick SW, Poston GJ, Folprecht G.Defined criteria for resectability improves rates of secondary resection after systemic therapy for liver limited metastatic colorectal cancer. Eur J Cancer. 2014 Jun;50(9):1590-601. doi: 10.1016/j.ejca.2014.02.024. Epub 2014 Mar 21.
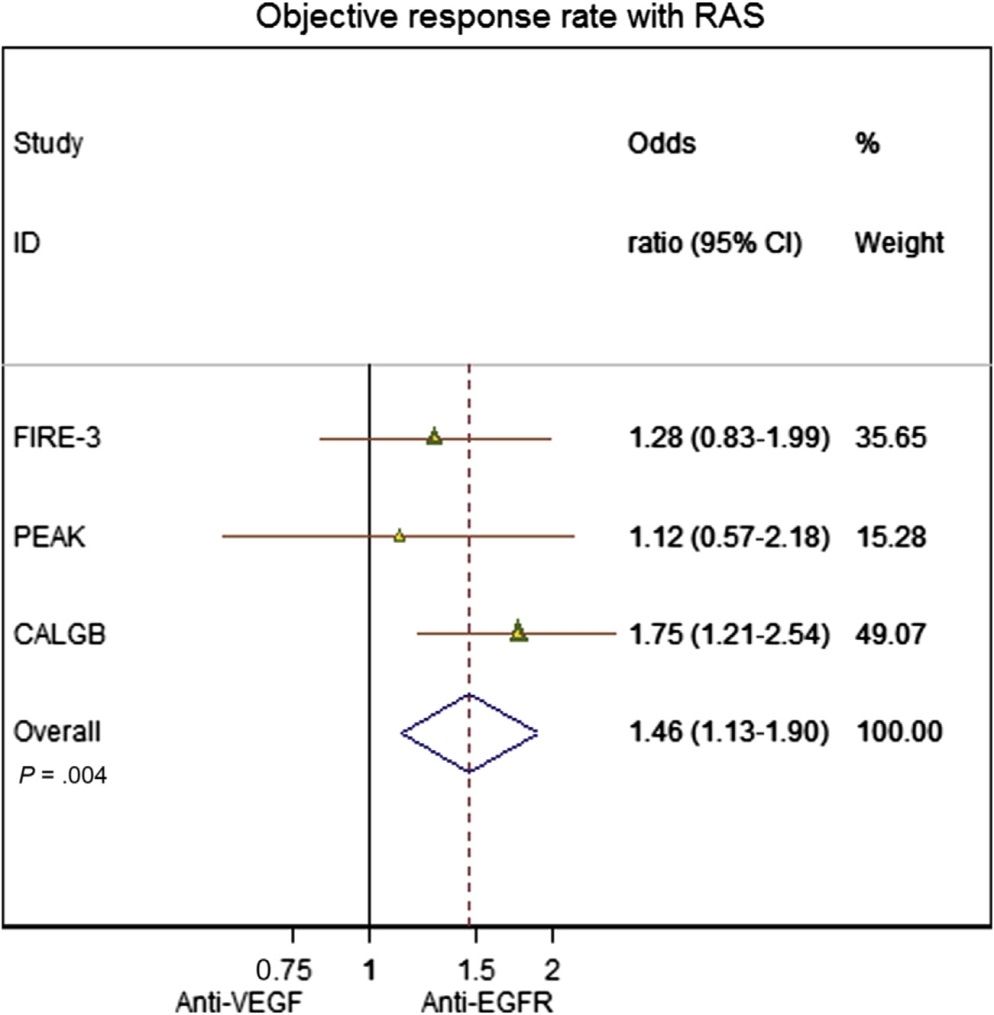
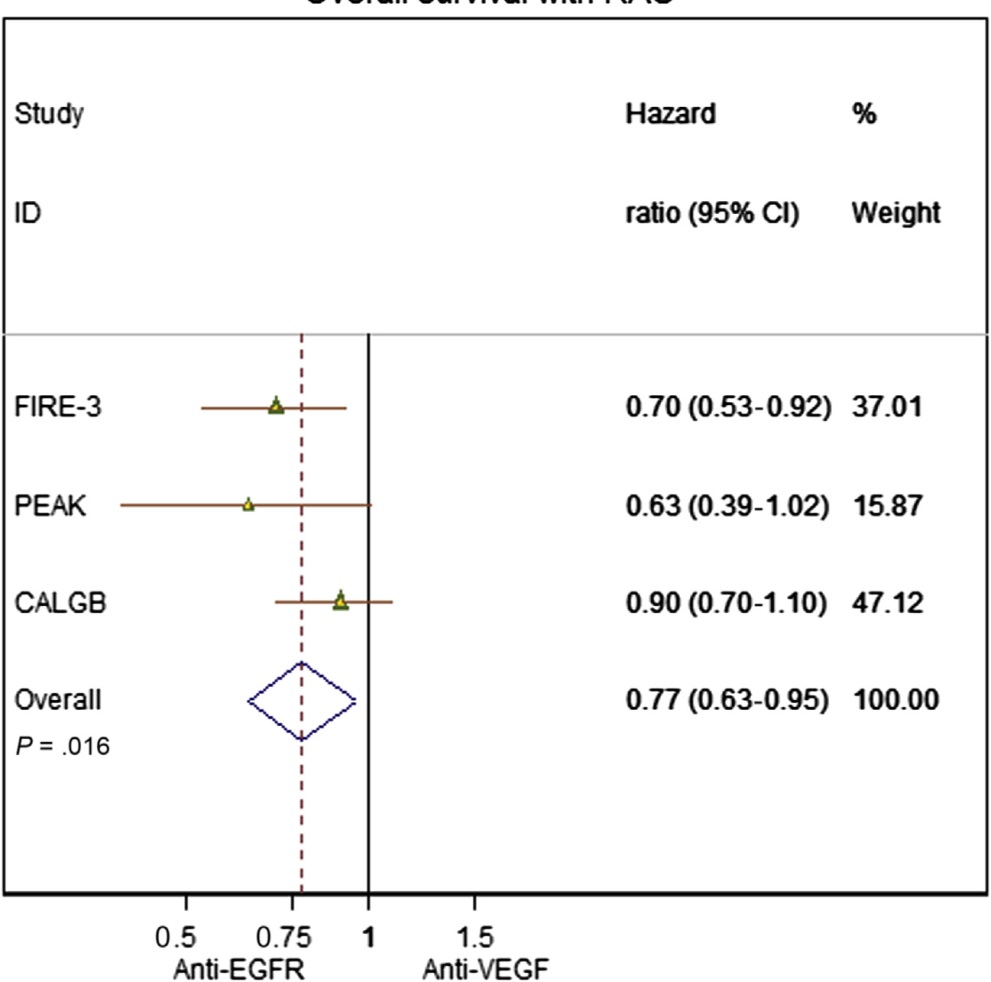
EGFR vs. VEGF plus chemo(TAS wt) 
Heinemann V at al FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014 Sep;15(10):1065-75. doi: 10.1016/S1470-2045(14)70330-4. Epub 2014 Jul 31.
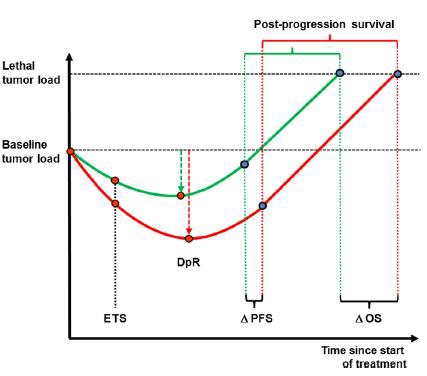
Heinemann V at al .Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2015 Sep;51(14):1927-36. doi: 10.1016/j.ejca.2015.06.116. Epub 2015 Jul 15.
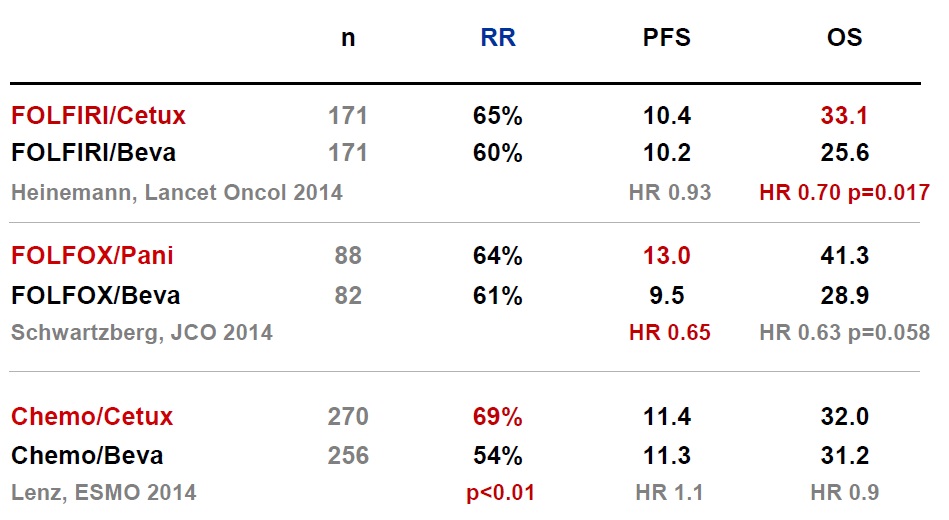 Heinemann V at al FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014 Sep;15(10):1065-75. doi: 10.1016/S1470-2045(14)70330-4. Epub 2014 Jul 31
Schwartzberg LS at al PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014 Jul 20;32(21):2240-7. doi: 10.1200/JCO.2013.53.2473. Epub 2014 Mar 31.
Lenz, ESMO 2014
Pooling the data… (RAS wild type)
Khattak MA, Martin H, Davidson A2, Phillips M.Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer. 2015 Jun;14(2):81-90.
EGFR vs. VEGF plus chemo(Resection: KRAS exon 2 wt)  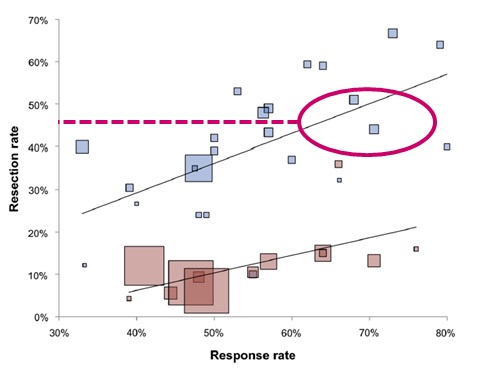 Venook, ASCO FOLFOXIRI combinations in first line therapy
动脉化疗与静脉化疗的比较
OPTILIV: i.a. FOLFOXIRI / cetuximab in pretreated patients
Levi et al, Ann Oncol 2016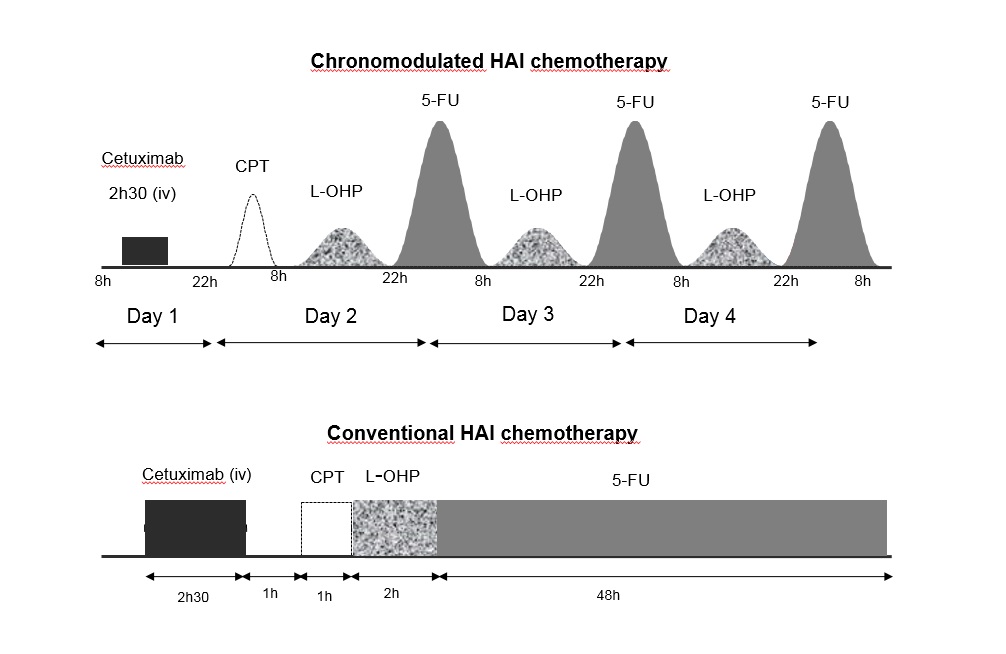 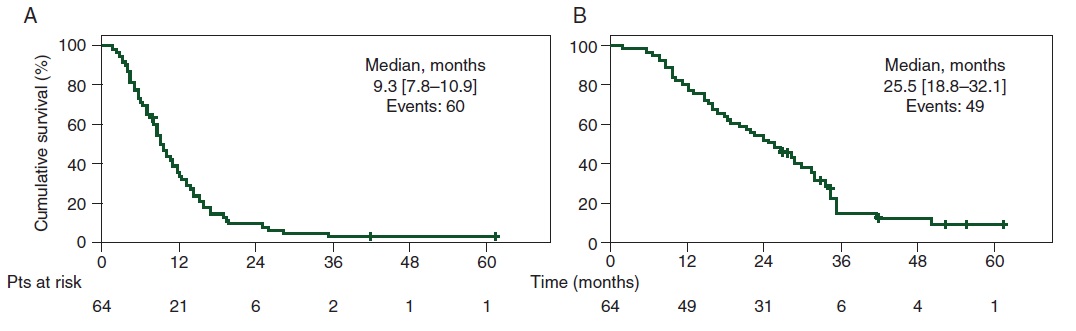 结论:The coordination of liver-specific intensive chemotherapy and surgery had a high curative intent potential that deserves upfront randomized testing.
Lévi FA at al Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol. 2016 Feb;27(2):267-74. doi: 10.1093/annonc/mdv548. Epub 2015 Nov 16.
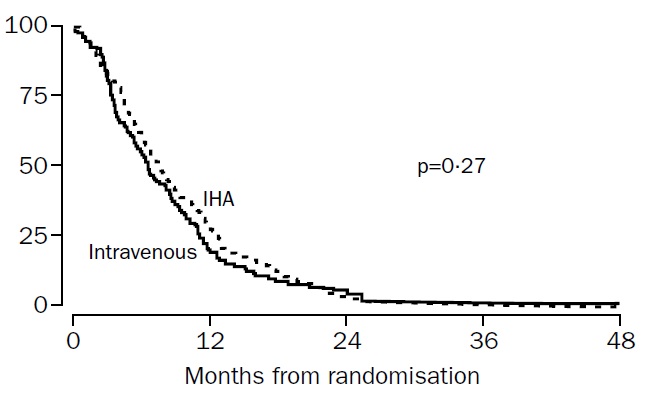
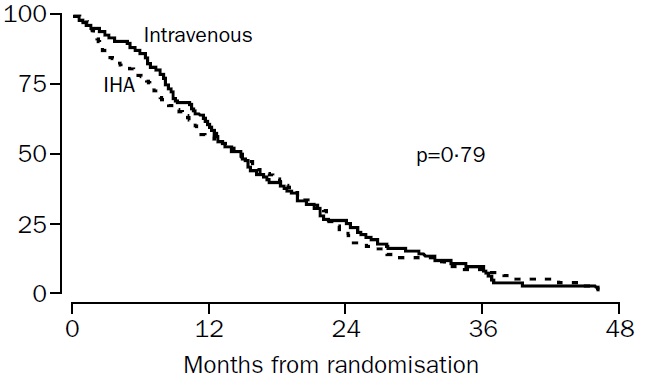
肝动脉化疗与静脉化疗治疗结直肠癌肝转移的比较(HAI vs. systemic chemotherapy) Kerr et al, Lancet 2003
Our results showed no evidence of an advantage in progression-free survival or overall survival for the IHA group; thus continued use of this regimen cannot be recommended outside of a clinical trial. (动脉灌注化疗与静脉化疗比较在PFS和OS上没有优势,不推荐临床实验以外的应用)
Kerr DJ at al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003 Feb 1;361(9355):368-73.
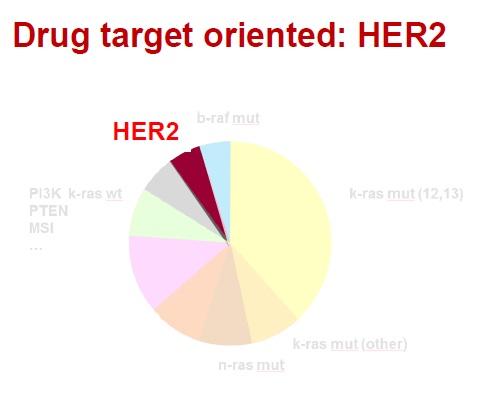
药物靶向治疗(Drug target oriented) Guinney et al Nature Medicine 2015 肿瘤内科学50年来在药物研制中的发展都是集中在细胞毒性攻击性的药物。虽然继蒽环类(阿霉素、表阿霉素)、铂类(顺铂、卡铂)之后又有很多强有力的化疗药物如泰素、泰索帝、开普拓、草酸铂、健择等问世并在各个不同的癌肿发挥重要的作用,但其性质仍然属于不能分辨肿瘤细胞和正常细胞的药物,临床应用受到诸多因素的限制。
进入二十一世纪的今天,分子靶向治疗(Molecular targeted therapy)已经不再是一个新名词。科学家们在不断探索癌症的分子生物学发病机理时,就意识到如果能够针对癌症的特异性分子变化给予有力的打击,将会大大改善治疗效果,引发抗癌治疗理念的变革。最近几年,新型分子靶向药物在临床实践中取得了显著的疗效,实践已表明了分子靶向治疗理论的正确性与可行性。把癌症的治疗推向了一个前所未有的新阶段。
上世纪末期,医学科学的不断发展,专业人士对恶性肿瘤的发病原因进行了深入的研讨,基因致癌的机理慢慢清晰起来,基于致癌基因的高端生物技术不断的被医学临床应用,分子靶向治疗,一种全新的治疗方法逐渐兴起。
One (few) target(s) Direct implication treatment Applicable across diseases?
一个(少数几个)靶向生物标志物阳性,暗示可以直接应用于某种疾病? 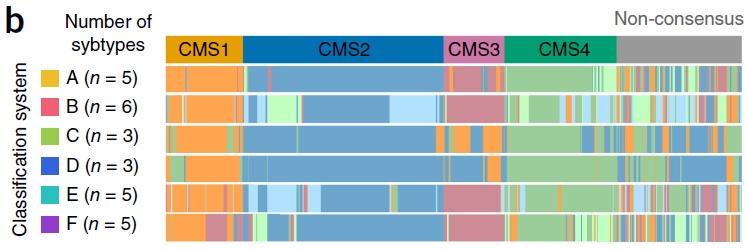 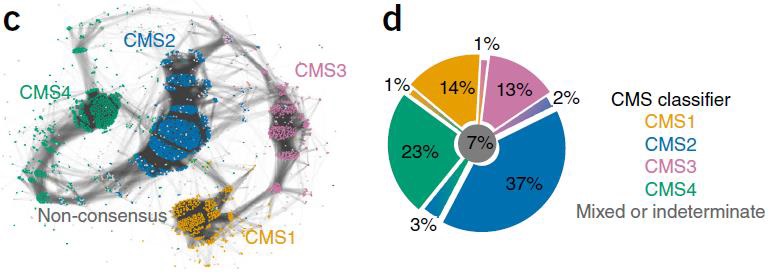
Guinney J at al The consensus molecular subtypes of colorectal cancer. Nat Med. 2015 Nov;21(11):1350-6. doi: 10.1038/nm.3967. Epub 2015 Oct 12.
Drug target oriented: BRAF
BRAF突变
BRAF背景介绍:
BRAF 是人类最重要的原癌基因之一, 大约8% 的人类肿瘤发生BRAF 突变。BRAF 绝大部分突变形式为BRAFV600E 突变, 主要发生于黑色素瘤、结肠癌和甲状腺癌中。该突变导致下游MEK-ERK 信号通路持续激活, 对肿瘤的生长增殖和侵袭转移至关重要, 是抗黑色素瘤等V600E 突变肿瘤的有效作用靶标之一。2011 年,首个BRAFV600E 靶向抑制剂威罗菲尼被FDA 批准上市, 用于治疗BRAFV600E 突变的晚期黑色素瘤患者, 有效延长了患者无进展生存期及总生存期, 取得了突破性的治疗效果, 也是典型的基于基因诊断选择用药的靶向治疗药物。但是耐药性的出现使得药物治疗效果受到限制, 其耐药机制、新药物开发以及预防或延缓耐药的研究成为目前需要解决的关键问题
BRAF抑制剂:
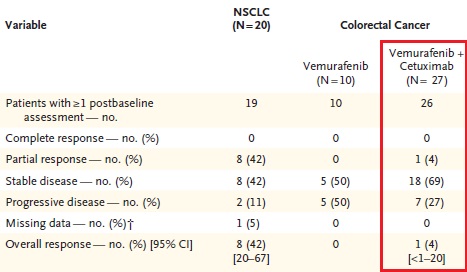
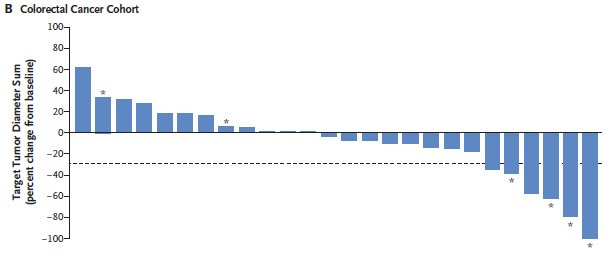
目前已发现两大类BRAF 抑制剂,一类是广谱的RAF 激酶抑制剂, 对RAF 各亚型、其他激酶如KIT、血管内皮生长因子受体 (VEGFR) 等也有抑制。其代表为索拉菲尼 (sorafenib)、RAF-265、XL-281 等。这类抑制剂具有广谱的抗肿瘤及抗血管生成作用, 抗肿瘤治疗一般不需要限定BRAF 基因的突变状态, 对于黑色素瘤尤其是携带BRAF 突变的恶性黑色素瘤患者目前尚没有明显临床疗效的报道。另一类是BRAFV600E 抑制剂, 对BRAF 尤其是BRAFV600E 有很高的抑制活性, 代表为威罗菲尼(vemurafenib, PLX4032) 和GSK2118436 (dabrafenib),威罗菲尼对BRAFV600E 突变的黑色素瘤患者具有明显的临床效果。
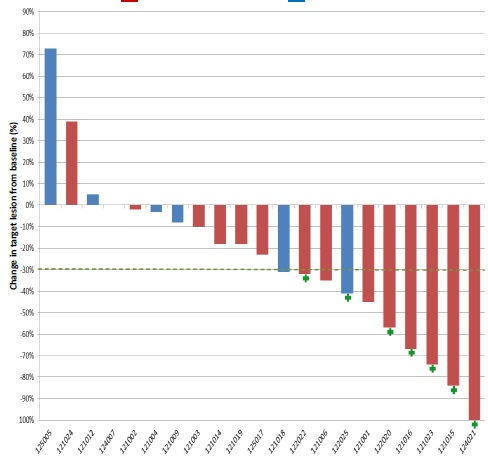
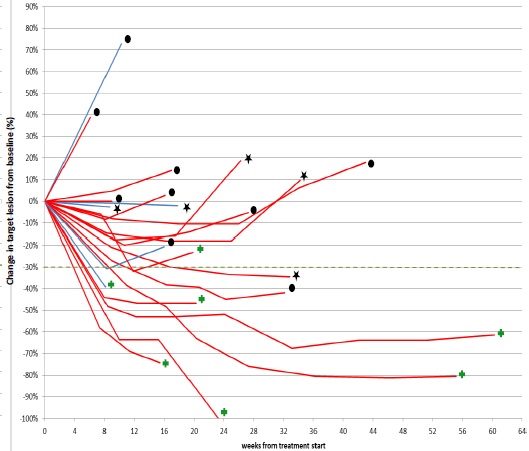
    Response to Trastuzumab/Lapatinib by HER2 IHC Score Siena, ASCO 2015
 *3 patients are not shown: 122026 (IHC 2+,not yet assessed); 121011 (IHC 3+) and 121013 (IHC 3+) early clinical PDs
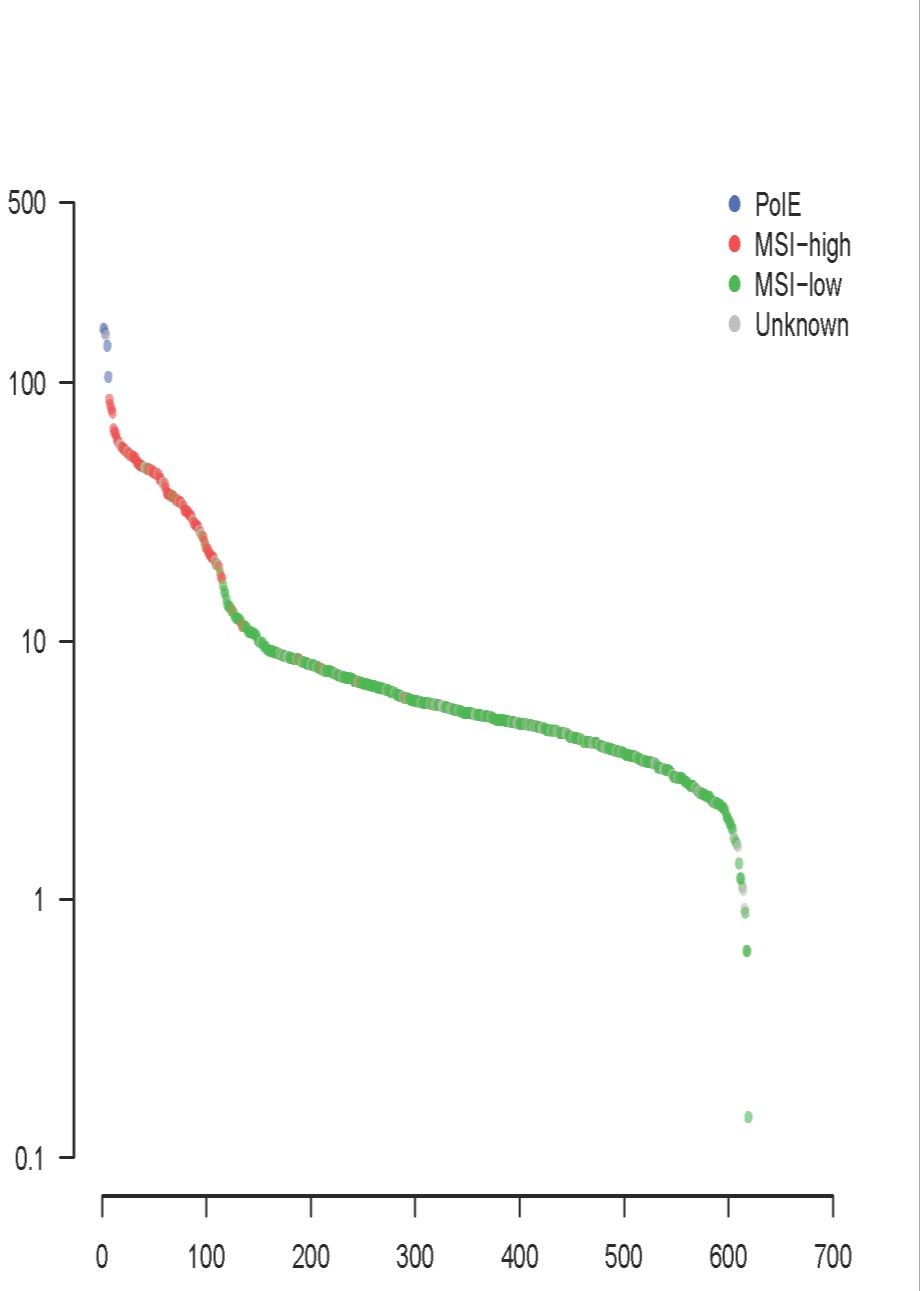
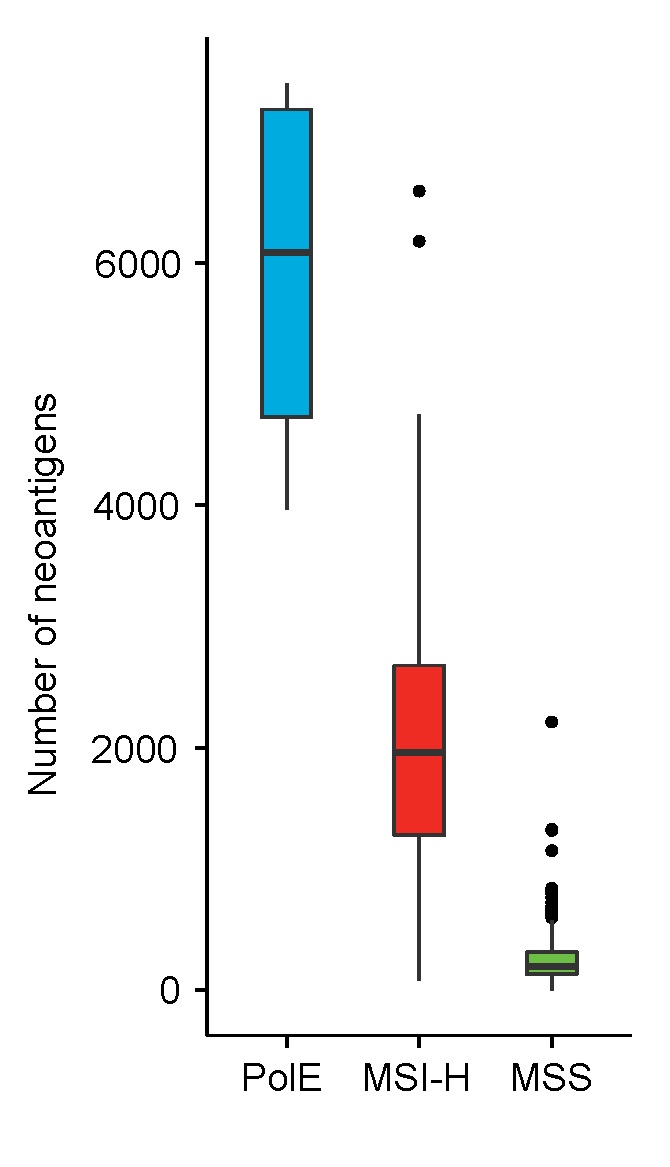
Microsatellite instable tumors(微卫星不稳定肿瘤): hypermutated, more neoantigens
Giannakis, ASCO 2015
Comprehensive molecular characterization of colorectal cancer reveals genomic predictors of immune cell infiltrates.   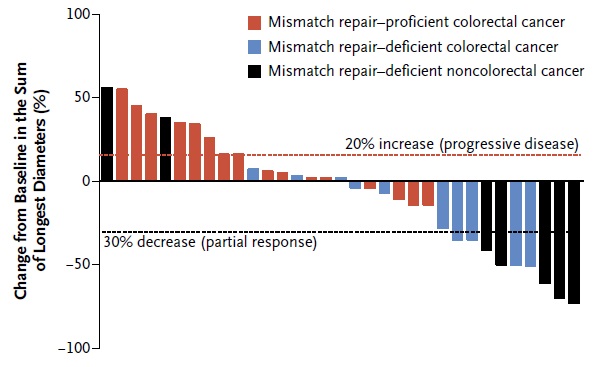
二、结肠癌肝转移介入治疗的现状和角色: 传统结直肠癌的围手术治疗包括 肝转移癌的新辅助治疗、肝转移癌的辅助治疗、肝转移癌的转化治疗、肝转移癌围手术期化疗(新辅助+辅助)以及肝转移癌的姑息化疗治疗。在这些传统治疗方法中,介入治疗究竟可以或已经扮演什么样的角色?定义如何?结果又如何呢?先看定义。 1. 结直肠肝转移癌经导管治疗新辅助治疗(Neoadjuvant):可一期切除的肝转移癌,外科术前进行经导管肝动脉灌注化疗。 术前治疗预防结肠癌肝转移,即结肠癌切除前进行肝动脉或结肠癌供血动脉的灌注化疗,所谓新辅助治疗。它应该还包括单独介入灌注化疗,全身化疗的联合和单一治疗间的对比,以及结肠癌切除术围手术期的化疗对比,如术前化疗+术后化疗 vs 单一术前化疗等。目的是减少肝转移癌发生率或推迟肝转移癌发生的时间,或改变肝转移癌发生的形式,提高结直肠癌手术切除的生存率。(chemotherapy given to facilitate surgery) 2. 结直肠肝转移癌经导管辅助治疗(adjuvantive):是指可一期切除的肝转移癌,外科切除术后进行经导管肝动脉灌注化疗(chemotherapy post surgery) 3. 结直肠肝转移癌经导管转化治疗(conversion therapies):经导管肝动脉灌注后,将不可一期切除的肝转移癌变成可一期切除的肝转移癌(down staging)。 4. 结直肠癌肝转移经导管围手术期化疗:对于可切除肝转移癌进行外科术前、术后经导管化疗 5. 结直肠癌肝转移癌的经导管姑息治疗:对永久不可切除的肝转移癌进行经导管化疗。
结直肠肝转移病人预后差。只有少于25%的患者有机会进行治愈性切除或经皮消融,他们其中70%的患者治疗后3年内消逝【1】。
经静脉全身化疗首选5-FU联合伊立替康或奥沙利铂和单克隆抗体的治疗反应率为31-62%,中位无进展期(Progression free survival,PFS)为6.9-10.6个月,中位整体生存率(overall survival,OS)14-21.5个月【2-5】。但是,对一线治疗不敏感的患者,二线或三线全身治疗疗效也不好,RR 4-21%,中位PFS 2.5-4.8【6-8】。
尽管基于导管治疗肝脏肿瘤有许多优势,并且在过去10年越来越多地在临床得到应用。但当与全身化疗和最好的支持治疗比较,仍然没有证据表明结直肠癌肝转移患者经肝动脉经泵灌注化疗(HAIC),或隔离循环化疗(isolated hepatic perfusion ),经肝动脉栓塞治疗(TACE)或经肝动脉放射治疗(TARE)治疗后可以分别提高提高生存率和改善PFS 。但是这些治疗的局部反应率和转化到可切除性一直以来显示可能对拯救病人(salvage patients) 。
2007年10个RCT研究表明肝动脉灌注化疗与全身化疗比较,并不改善病人的生存率【9】。但是,对于一线治疗失败的患者,肝动脉灌注化疗联合全身治疗可以改善病人无进展生存期4-7个月【10-12】。转化为可切除性转移癌达到35-47%【11,13】。
静脉化疗后进展的病人,隔离式循环化疗局部反应率可以达到60-80%,PFS可以达到12个月【14,15】。
1998年开始,有几个临床试验用于评价对静脉化疗耐受的肝转移癌病人进行常规TACE。这些研究的大多数,联合顺铂、表阿霉素和丝裂霉素和PVA进行栓塞治疗,但是方案差异程度大,带来是TACE术后结果的差异,报告客观反应率2-63%,PFS 为3-8个月,OS 8.6-14.3个月【16-20】。仅有有限的资料可以决定亚分组病人将从这一治疗获益。
TACE前进行一线或二线治疗的病人比较3-5线静脉化疗(中位生存期6个月;P=0.03)【20】后的病人似乎有较好的结果(中位生存期 11-12个月)。存在肝外转移还不清楚对生存率的影响。大样本病人报告的客观反应率低于15%【19,20】。包括晚期肿瘤肝侵犯研究显示首次TACE术后的整体生存率低于10个月【16,18,20】。
药物洗脱微球可以提供有控制的药物释放到肿瘤,减少静脉化疗对全身的副作用,并改善TACE 可重复性。伊立替康对于治疗结直肠癌肝转移是一种强有力的药物,体内高清除率和高肝分泌率,适合经动脉肝治疗。
今天,各种载药微球应用于临床,如荷载伊立替康的微球。用伊立替康载药微球TACE有六个回顾性研究,一个215例随机对照研究【21~27】。
如果病人事先进行静脉化疗,局部肿瘤控制(无进展)是40-86%;然而首次DEB-TACE术后,PFS和 OS分别是有限的4-8.1个月和5.4-13.3个月。 应用荷载伊立替康微球的经导管栓塞,可以在相当的比例下获得结直肠癌肝转移病灶血管几近消失。据报告【25】,使用伊立替康荷载的微球栓塞,肝转移病变经增强CT扫描,在第三个月染色完全消失的为7/29例,肿瘤容积≥50%肿瘤坏死为14/29%。在药物、栓塞剂和微球方面,选择与非选择应用没有标准。
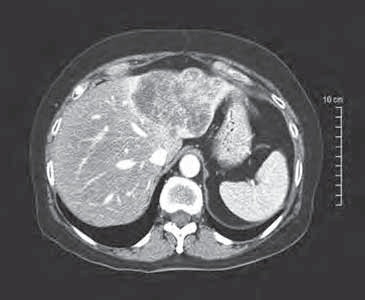
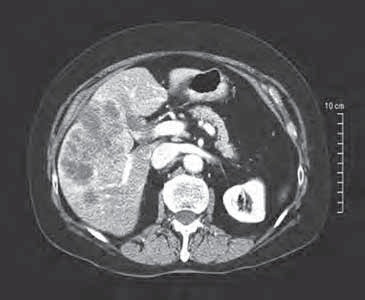
术前:肝左右叶肝转移癌
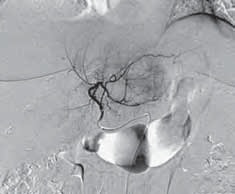
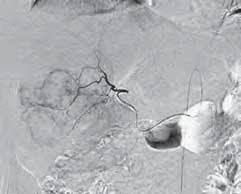
伊立替康荷载微球栓塞
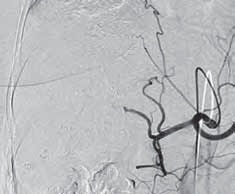
两次TACE术后随访:首次TACE术后病人存活12个月
应用伊立替康荷载药物洗脱微球进行结直肠癌肝转移的治疗,有报告可以包括两叶动脉的同时栓塞,间隔3-8周时间进行下一次栓塞。考虑到转移性疾病的播散“叶动脉栓塞(lobar treatment)似乎是必须的。但是如果术前影像显示肿瘤大部分在有限肝段内,经导管治疗可以在叶动脉注射前进行选择性肝段动脉注射以增加潜在的疗效。这一技术应用在30/74例的研究中并没有并发症的增加【25】。
经动脉导管放射性栓塞治疗结直肠癌肝转移已经有7个临床试验对1174个病人进行了评价,其中605例的资料来自于多中心的研究【29-34】。多数病人在TARE之前进行了静脉全身化疗。取决于评估的时间和肿瘤的容积,局部肿瘤控制率在29%-73%之间。整体生存率在有限的6.2~11.6之间。
结论:肝动脉灌注化疗(HAIC)、隔离循环化疗(IHP)、颈动脉化疗性栓塞(TACE)以及经动脉放疗性栓塞(TARE)对于一线或N线标准姑息性静脉全身化疗失败的病人是个治疗选择。这些治疗的临床结果仍然是有限的,取决于肿瘤负荷、临床状态和肝外转移播散,PFS延长在4-8月之间。DEB-TACE的优点是高水平的标准化治疗和手术操作简单。
三、结肠癌肝转移介入治疗的未来
结直肠癌联合治疗(Multimodality Management)包括
References:
1. Pwint TP, Midgley R, Kerr DJ. Regional hepatic chemotherapies in the treatment of colorectal cancer metastases to the liver. Semin Oncol. 2010 Apr;37(2):149-59.
2. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000 Sep 28;343(13):905-14.
3. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer(免费). J Clin Oncol. 2004 Jan 1;22(1):23-30. Epub 2003 Dec 9.
4. Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L, Borsellino N, Misino A, Romito S, Durini E, Cordio S, Di Seri M, Lopez M, Maiello E, Montemurro S, Cramarossa A, Lorusso V, Di Bisceglie M, Chiarenza M, Valerio MR, Guida T, Leonardi V, Pisconti S, Rosati G, Carrozza F, Nettis G, Valdesi M, Filippelli G, Fortunato S, Mancarella S, Brunetti C; Gruppo Oncologico Dell'Italia Meridionale. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale(免费). J Clin Oncol. 2005 Aug 1;23(22):4866-75. Epub 2005 Jun 6.
5. Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L, Jaffe C, Rubinstein L, Zwiebel J, Kaplan RS. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301(免费). J Clin Oncol. 2006 Jul 20;24(21):3354-60.
6. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer(free). N Engl J Med. 2004 Jun 3;350(23):2335-42.
7. Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, Le Bail N, Haller DG Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003 Jun 1;21(11):2059-69.
8. Park ER, Li FP, Liu Y, Emmons KM, Ablin A, Robison LL, Mertens AC; Childhood Cancer Survivor Study. Health insurance coverage in survivors of childhood cancer: the Childhood Cancer Survivor Study(FREE). J Clin Oncol. 2005 Dec 20;23(36):9187-97.
9. Mocellin S1, Pilati P, Lise M, Nitti D. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol. 2007 Dec 10;25(35):5649-54.
10. Boige V, Malka D, Elias D, Castaing M, De Baere T, Goere D, Dromain C, Pocard M, Ducreux M. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008 Jan;15(1):219-26. Epub 2007 Sep 26.
11.Samaras P, Breitenstein S, Haile SR, Stenner-Liewen F, Heinrich S, Feilchenfeldt J, Renner C, Knuth A, Pestalozzi BC, Clavien PA. Selective intra-arterial chemotherapy with floxuridine as second- or third-line approach in patients with unresectable colorectal liver metastases. Ann Surg Oncol. 2011 Jul;18(7):1924-31. doi: 10.1245/s10434-010-1505-2. Epub 2011 Jan 5.
12. Tsimberidou AM, Vaklavas C, Fu S, Wen S, Lim JA, Hong D, Wheler J, Naing A, Uehara C, Wallace M, Kurzrock R. Hepatic arterial infusion therapy in advanced cancer and liver-predominant disease: the MD Anderson Experience. Hepatogastroenterology. 2013 Oct;60(127):1611-23.
13. DʼAngelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF, Capanu M, Kingham TP, Fong Y, DeMatteo RP, Allen PJ, Jarnagin WR, Kemeny N. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015 Feb;261(2):353-60.
14. Reddy SK, Kesmodel SB, Alexander HR Jr. Isolated hepatic perfusion for patients with liver metastases. Ther Adv Med Oncol. 2014 Jul;6(4):180-94. doi: 10.1177/1758834014529175.
15. Magge D, Choudry HA, Zeh HJ 3rd, Cunningham DE, Steel J, Holtzman MP, Jones HL, Pingpank JF, Bartlett DL, Zureikat AH. Outcome analysis of a decade-long experience of isolated hepatic perfusion for unresectable liver metastases at a single institution. Ann Surg. 2014 May;259(5):953-9. doi: 10.1097/SLA.0000000000000261.
16.Tellez C, Benson AB 3rd, Lyster MT, Talamonti M, Shaw J, Braun MA, Nemcek AA Jr, Vogelzang RL.Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998 Apr 1;82(7):1250-9.
17. Leichman CG, Jacobson JR, Modiano M, Daniels JR, Zalupski MM, Doroshow JH, Fletcher WS, Macdonald JS. Hepatic chemoembolization combined with systemic infusion of 5-fluorouracil and bolus leucovorin for patients with metastatic colorectal carcinoma: A Southwest Oncology Group pilot trial. Cancer. 1999 Sep 1;86(5):775-81.
18. Hong K, McBride JD, Georgiades CS, Reyes DK, Herman JM, Kamel IR, Geschwind JF. Salvage therapy for liver-dominant colorectal metastatic adenocarcinoma: comparison between transcatheter arterial chemoembolization versus yttrium-90 radioembolization. J Vasc Interv Radiol. 2009 Mar;20(3):360-7. doi: 10.1016/j.jvir.2008.11.019. Epub 2009 Jan 23.
19. Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study(FREE). Radiology. 2009 Jan;250(1):281-9. doi: 10.1148/radiol.2501080295.
20.Albert M, Kiefer MV, Sun W, Haller D, Fraker DL, Tuite CM, Stavropoulos SW, Mondschein JI, Soulen MC. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer. 2011 Jan 15;117(2):343-52. doi: 10.1002/cncr.25387. Epub 2010 Sep 9.
21. Fiorentini G, Aliberti C, Turrisi G, Del Conte A, Rossi S, Benea G, Giovanis P. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: results of a phase II clinical study ( FREE). In Vivo. 2007 Nov-Dec;21(6):1085-91.
22. Martin RC, Joshi J, Robbins K, Tomalty D, Bosnjakovik P, Derner M, Padr R, Rocek M, Scupchenko A, Tatum C. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011 Jan;18(1):192-8. doi: 10.1245/s10434-010-1288-5. Epub 2010 Aug 26.
23.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V, Coschiera P. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study (FREE). Anticancer Res. 2012 Apr;32(4):1387-95.
24. Narayanan G, Barbery K, Suthar R, Guerrero G, Arora G. Transarterial chemoembolization using DEBIRI for treatment of hepatic metastases from colorectal cancer. Anticancer Res. 2013 May;33(5):2077-83.
25. Huppert P, Wenzel T, Wietholtz H. Transcatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan-eluting microspheres in a salvage patient population. Cardiovasc Intervent Radiol. 2014 Feb;37(1):154-64. doi: 10.1007/s00270-013-0632-0. Epub 2013 May 14.
26. Stutz M, Mamo A, Valenti D, Hausvater A, Cabrera T, Metrakos P, Chaudhury P, Steacy G, Garoufalis E, Kavan P. Real-Life Report on Chemoembolization Using DEBIRI for Liver Metastases from Colorectal Cancer(FREE). Gastroenterol Res Pract. 2015;2015:715102. doi: 10.1155/2015/715102. Epub 2015 Feb 26.
27. Iezzi R, Marsico VA, Guerra A, Cerchiaro E, Cassano A, Basso M, Devicienti E, Rodolfino E, Barone C, Bonomo L.Trans-Arterial Chemoembolization with Irinotecan-Loaded Drug-Eluting Beads (DEBIRI) and Capecitabine in Refractory Liver Prevalent Colorectal Metastases: A Phase II Single-Center Study. Cardiovasc Intervent Radiol. 2015 Mar 24. [Epub ahead of print]
28.Cosimelli M1, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG, Perrone M, Giampalma E, Angelelli B, Fiore F, Lastoria S, Bacchetti S, Gasperini D, Geatti O, Izzo F; Italian Society of Locoregional Therapies in Oncology (SITILO). Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases (FREE). Br J Cancer. 2010 Jul 27;103(3):324-31. doi: 10.1038/sj.bjc.6605770. Epub 2010 Jul 13.
29. Kalva SP, Rana RS, Liu R, Rachamreddy N, Dave B, Sharma A, Ganguli S, Rabito C, Kwak E, Blaszkowsky LS. Yttrium-90 Radioembolization as Salvage Therapy for Liver Metastases From Colorectal Cancer. Am J Clin Oncol. 2014 Nov 4. [Epub ahead of print]
30. Golfieri R, Mosconi C, Giampalma E, Cappelli A, Galaverni MC, Pettinato C, Renzulli M, Monari F, Mazzarotto R, Pinto C, Angelelli B. Selective transarterial radioembolisation of unresectable liver-dominant colorectal cancer refractory to chemotherapy. Radiol Med. 2015 Aug;120(8):767-76. doi: 10.1007/s11547-015-0504-6. Epub 2015 Feb 13.
31. Kennedy AS, Ball D, Cohen SJ, Cohn M, Coldwell DM, Drooz A, Ehrenwald E, Kanani S, Rose SC, Nutting CW, Moeslein FM, Savin MA, Schirm S, Putnam SG 3rd, Sharma NK, Wang EA. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol. 2015 Apr;6(2):134-42. doi: 10.3978/j.issn.2078-6891.2014.109.
32. Abbott AM, Kim R, Hoffe SE, Arslan B, Biebel B, Choi J, El-Haddad G, Kis B, Sweeney J, Meredith KL, Almhanna K, Strosberg J, Shibata D, Fulp WJ, Shridhar R. Outcomes of Therasphere Radioembolization for Colorectal Metastases. Clin Colorectal Cancer. 2015 Sep;14(3):146-53. doi: 10.1016/j.clcc.2015.02.002. Epub 2015 Feb 16.
33. Sabet A, Meyer C, Aouf A, Sabet A, Ghamari S, Pieper CC, Mayer K, Biersack HJ, Ezziddin S. Early post-treatment FDG PET predicts survival after 90Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur J Nucl Med Mol Imaging. 2015 Mar;42(3):370-6. doi: 10.1007/s00259-014-2935-z. Epub 2014 Oct 29.
34. Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients.Ann Surg Oncol. 2015 Mar;22(3):794-802. doi: 10.1245/s10434-014-4164-x. Epub 2014 Oct 17.
(责任编辑:Mr.Editor) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

|